The Maillard reaction in coffee is a caramelization process in which sugars combine with amino acids, peptides and proteins under high temperatures.
This process is very important for the final quality of the coffee. It is through it that the grain acquires its brown color and its characteristic aromas.
This reaction was first described by the French chemist Louis Camille Maillard in 1912. In this reaction also occurs the formation of volatile compounds responsible for the aroma. When carbohydrates and amino acids are broken down, dark colored compounds called malloids are formed.
Roasting processes.
Green coffee roasting takes place at temperatures above 180c, where flavors and aromas develop. Structural changes also occur in the grain, involving many interactions between compounds.
During roasting, heat from the roaster is transferred to the beans. With the internal increase in temperature, the evaporation of moisture from the grains occurs in a process called Endothermic. As the temperature increases, exothermic reactions arise, at the same time with the formation of volatile compounds and CO2. As the internal pressure increases, the grains expand, causing small cracks in their walls. This event is called Crack.
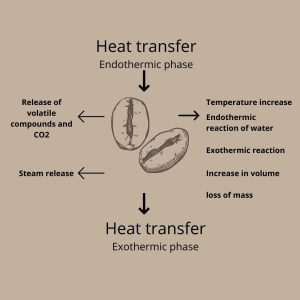
Physico-Chemical Changes.
The roasting process, which is highly complex, involves chemical as well as physical reactions. These reactions occur at temperatures above 200°C. Chemical Changes.
The main carbohydrate present in green beans is sucrose. During roasting, sucrose is degraded and a small amount remains in the roasted grain. This residual sucrose undergoes a process of pyrolysis (decomposition of materials by the action of high temperatures. The breakage of the molecular structure of the grain occurs by the action of heat), and caramelization. Then it is hydrolyzed into glucose and fructose, within the Maillard reaction, which gives the coffee its color and flavor. Other compounds that also change are chlorogenic acids, lipids and other nitrogen-containing compounds.
Physical Changes
The physical change starts with the color of the grain, which goes from green to brown. This color depends on the chosen roasting point. With dehydration and pyrolysis, the grain suffers a mass loss of 14% to 20%. The darker the roast, the greater the loss. Usually in medium roasts the loss is 15%.
1kg of green coffee = 850g of roasted coffee.
Another change is the increase in grains due to the formation of CO2 from the steam and volatile compounds. The grain volume can reach 40% more, while the density decreases. Cytoplasms (porosities) are also formed where compounds responsible for flavor, carbohydrates, lipids, proteins, minerals and caffeine and chlorogenic acids are installed.
Studies suggest that the antioxidants present in coffee could be linked to caffeine or chlorogenic acid found in green beans. But studies show that the Maillard reaction is the main source of antioxidants. But on the other hand, coffee beans lose 90% of the chlorogenic acid during the roasting process.
That’s why green coffee infusion is known for its high content of chlorogenic acid and its benefits.
Try our Green Coffee Infusion or Medium Roast Coffee!





